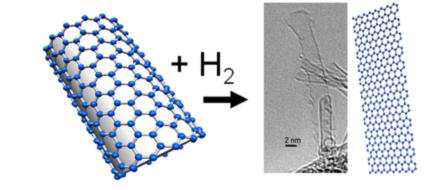
Reaction of single-walled carbon nanotubes (SWNTs) with hydrogen gas. (Credit: Image courtesy of Umeå University)
ScienceDaily (May 10, 2011) — An international research team has discovered a new method to produce belts of graphene called nanoribbons. By using hydrogen, they have managed to unzip single-walled carbon nanotubes. The method also opens the road for producing nanoribbons of graphane, a modified and promising version of graphene.
A thin flake plain carbon, just one atom thick, became world famous last year. The discovery of the super material graphene gave Andre Geim and Konstantin Novoselov the Nobel Prize in Physics 2010. Graphene has a wide range of unusual and highly interesting properties. As a conductor of electricity it performs as well as copper. As a conductor of heat it outperforms all other known materials.
There are possibilities to achieve strong variations of the graphene properties for instance by making graphene in a form of belts with various width, so called nanoribbons. Nanoribbons were prepared for the first time two years ago. A method to produce them is to start from carbon nanotubes and to use oxygen treatment to unzip into nanoribbons. However, this method leaves oxygen atoms on the edges of nanoribbons, which is not always desirable.
In the new study the research team shows that it is also possible to unzip single-walled carbon nanotubes by using a reaction with molecular hydrogen. Nanoribbons produced by the new method will have hydrogen on the edges and this can be an advantage for some applications. Alexandr Talyzin, physicist at Umeå University in Sweden, has over the past decade been studying how hydrogen reacts with fullerenes, which are football-shaped carbon molecules.
"Treating the carbon nanotubes with hydrogen was a logical extension of our research. Our previous experience has been of great help in this work," says Alexandr Talyzin.
Nanotubes are typically closed by semi-spherical cups, essentially halves of fullerene molecules. The researchers have previously proved that fullerene molecules can be completely destroyed by very strong hydrogenation. Therefore, they expected similar results for nanotube end cups and tried to open the nanotubes by using hydrogenation. The effect was indeed confirmed and they also managed to reveal some other exciting effects.
The most interesting discovery was that some carbon nanotubes were unzipped into graphene nanoribbons as a result of prolonged hydrogen treatment. What is even more exciting -- unzipping of nanotube with hydrogen attached to the side walls could possibly lead to synthesis of hydrogenated graphene: graphane. So far, graphane was attempted to be synthesized mostly by reaction of hydrogen with graphene. This appeared to be very difficult, especially if the graphene is supported on some substrate and only one side is available for the reaction. However, hydrogen reacts much easier with the curved surface of carbon nanotubes.
"Our new idea is to use hydrogenated nanotubes and unzip them into graphane nanoribbons. So far, only the first step towards graphane nanoribbon synthesis is done and a lot more work is required to make our approach effective," explains Alexandr Talyzin. "Combined experience and expertise from several groups at different universities, was a key to success."

