Theoretically, the electrical properties of carbon nanotubes are predicted to surpass those of copper – a well-known electrical conductor – in both conductivity and current density. The electronic structure is partially responsible for this. For example, metals have no gap in their electronic structure separating valence and conduction bands, while semiconductor materials such as carbon do have a band gap. Reporting in Nanotechnology, researchers perform a comprehensive study of the electronic properties of zigzag-zigzag double-walled carbon nanotubes (DWCNTs).
Example of a DWCNT molecule.
Carbon nanotubes were originally modelled as two-dimensional (2D) folded graphene sheets. The theoretical calculations predicted that 2/3 of all zigzag single-walled carbon nanotubes (SWCNTs) would have a band gap and thus be considered semiconductors. The other 1/3 would be metallic.
Similarly, multi-walled carbon nanotubes (two tubes or more) were predicted to be metallic if any of their constituent tubes were considered metallic in that model. However, the models do not take into account any orbital hybridization due to curvature and interaction between the tubes.
Effect of orbital hybridization
Here, the researchers include the effect that orbital hybridization has on the properties of SWCNTs and apply that to the study of zigzag-zigzag DWCNTs. The tubes are modelled at the atomic level and the molecule formed is further reduced to a single unit cell. It is virtually repeated as an infinite chain. This method helps save computer processing time when performing computational simulations using density functional theory (DFT).
Two semiconductor tubes
The results of the computational simulations are grouped according to the properties originally assigned to SWCNTs from the theoretical calculations. DWCNTs with both inner and outer semiconductor (s@s) tubes have a band gap equal or less than those of their constituent SWCNTs: between 0.3 and 0.5 eV. This change can be attributed to the interaction between the inner and outer tubes.
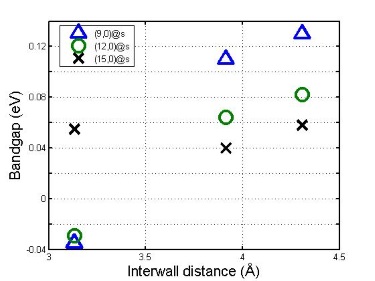
Electronic band gap as a function of interwall distance for m@s DWCNTs.
A semiconductor tube and a metallic tube
Combining a semiconductor inner tube with a metallic outer tube (s@m) results in a much smaller band gap than s@s DWCNTs: ranging from a 0.066 overlap to 0.078 eV. In this case, combining a metallic tube with a semiconductor inner tube does not guarantee metallic behaviour for the DWCNT.
Two metallic tubes
Even when combining two metallic inner and outer tubes (m@m) the resulting DWCNT band gap is not zero or overlapping. The reason is that the constituent tubes themselves show a small band gap when calculated using a more complex model. This is despite them being considered metallic from the theoretical calculations.
Although further reduced when combined in a DWCNT configuration, for the three cases studied a band gap remains. Most interesting is the case of a metallic inner tube and semiconductor outer tube (m@s) in a DWCNT configuration. When the inner tube has a diameter less than 10 Å the band gap increases with increasing interwall distance. Otherwise it remains unaffected by the interwall distance (above).
Categorization of DWCNTs and determination of their properties helps identify which of these molecules are best suited for certain applications. For example, transistors where band-gap tuning properties are desired, or macroscopic carbon nanotube conducting wires where metallic-like behaviour is most suitable.


